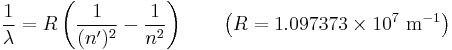Hydrogen spectral series
The emission spectrum of atomic hydrogen is divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed spectral lines are due to electrons moving between energy levels in the atom. The spectral series are important in astronomy for detecting the presence of hydrogen and calculating red shifts. Further series were discovered as spectroscopy techniques developed.
Contents |
Physics
In physics, the spectral lines of hydrogen correspond to particular jumps of the electron between energy levels. The simplest model of the hydrogen atom is given by the Bohr model. When an electron jumps from a higher energy to a lower, a photon of a specific wavelength is emitted.
The spectral lines are grouped into series according to n'. Lines are named sequentially starting from the longest wavelength/lowest frequency of the series, using Greek letters within each series. For example, the 2 → 1 line is called "Lyman-alpha" (Ly-α), while the 7 → 3 line is called "Paschen-delta" (Pa-δ). Some hydrogen spectral lines fall outside these series, such as the 21 cm line; these correspond to much rarer atomic events such as hyperfine transitions.[1] The fine structure also results in single spectral lines appearing as two or more closely grouped thinner lines, due to relativistic corrections.[2] Typically one can only observe these series from pure hydrogen samples in a lab. Many of the lines are very faint and additional lines can be caused by other elements (such as helium if using sunlight, or nitrogen in the air). Lines outside of the visible spectrum typically cannot be seen in observations of sunlight, as the atmosphere absorbs most infra-red and ultraviolet wavelengths.
Rydberg formula
The energy differences between levels in the Bohr model, and hence the wavelengths of emitted/absorbed photons, is given by the Rydberg formula[3]:
where n is the initial energy level, n′ is the final energy level, and R is the Rydberg constant.[4] Meaningful values are returned only when n is greater than n′ and the limit of one over infinity is taken to be zero.
Series
All wavelengths are given to 3 significant figures.
Lyman series (n′ = 1)
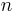 |
λ (nm) |
|---|---|
| 2 | 122 |
| 3 | 103 |
| 4 | 97.3 |
| 5 | 95.0 |
| 6 | 93.8 |
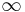 |
91.2 |
The series is named after its discoverer, Theodore Lyman, who discovered the spectral lines from 1906-1914. All the wavelengths in the Lyman series are in the ultraviolet band.[5][6]
Balmer series (n′ = 2)
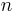 |
λ (nm) |
|---|---|
| 3 | 656 |
| 4 | 486 |
| 5 | 434 |
| 6 | 410 |
| 7 | 397 |
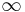 |
365 |
Named after Johann Balmer, who discovered the Balmer formula, an empirical equation to predict the Balmer series, in 1885. Balmer lines are historically referred to as "H-alpha", "H-beta", "H-gamma" and so on, where H is the element hydrogen.[7] Four of the Balmer lines are in the technically "visible" part of the spectrum, with wavelengths longer than 400 nm. Parts of the Balmer series can be seen in the solar spectrum. H-alpha is an important line used in astronomy to detect the presence of hydrogen.
Paschen series (n′ = 3)
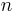 |
λ (nm) |
|---|---|
| 4 | 1870 |
| 5 | 1280 |
| 6 | 1090 |
| 7 | 1020 |
| 8 | 954 |
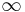 |
820 |
Named after the Austro-German physicist Friedrich Paschen who first observed them in 1908. The Paschen lines all lie in the infrared band.[8]
Brackett series (n′ = 4)
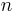 |
λ (nm) |
|---|---|
| 5 | 4050 |
| 6 | 2630 |
| 7 | 2170 |
| 8 | 1940 |
| 9 | 1820 |
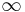 |
1460 |
Named after the American physicist Frederick Sumner Brackett who first observed the spectral lines in 1922.[9]
Pfund series (n′ = 5)
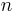 |
λ (nm) |
|---|---|
| 6 | 7460 |
| 7 | 4650 |
| 8 | 3740 |
| 9 | 3300 |
| 10 | 3040 |
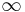 |
2280 |
Experimentally discovered in 1924 by August Herman Pfund.[10]
Humphreys series (n′ = 6)
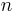 |
λ (nm) |
|---|---|
| 7 | 12400 |
| 8 | 7500 |
| 9 | 5910 |
| 10 | 5130 |
| 11 | 4670 |
 |
3280 |
Discovered by American physicist Curtis J. Humphreys.[11]
Further (n′ > 6)
Further series are unnamed, but follow exactly the same pattern as dictated by the Rydberg equation. Series are increasingly spread out and occur in increasing wavelengths. The lines are also increasingly faint, corresponding to increasingly rare atomic events.
Extension to other systems
The concepts of the Rydberg formula can be applied to any system with a single particle orbiting a nucleus, for example a He+ ion or a muonium particle. The equation must be modified based on the system's Bohr radius; emissions will be of a similar character but at a different range of energies.
All other atoms possess at least two electrons in their neutral form and the interactions between these electrons makes analysis of the spectrum by such simple methods as described here impractical. The deduction of the Rydberg formula was a major step in physics, but it was long before an extension to the spectra of other elements could be accomplished.
See also
- Hydrogen line (21 cm)
- Astronomical spectroscopy
- Moseley's law
- Theoretical and experimental justification for the Schrödinger equation
- Lyman series
- Balmer series
References
- ^ "The Hydrogen 21-cm Line". Hyperphysics. Georgia State University. 2004-10-30. http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/h21.html. Retrieved 2009-03-18.
- ^ Liboff, Richard L. (2002). Introductory Quantum Mechanics. Addison-Wesley. ISBN 0-8053-8714-5.
- ^ Bohr, Niels (1985), "Rydberg's discovery of the spectral laws", in Kalckar, J., N. Bohr: Collected Works, 10, Amsterdam: North-Holland Publ., pp. 373–9
- ^ "CODATA Recommended Values of the Fundamental Physical Constants: 2006" (PDF). Committee on Data for Science and Technology (CODATA). NIST. http://physics.nist.gov/cuu/Constants/codata.pdf.
- ^ Lyman, Theodore (1906), "The Spectrum of Hydrogen in the Region of Extremely Short Wave-Length", Memoirs of the American Academy of Arts and Sciences, New Series 13 (3): 125–146, ISSN 0096-6134, JSTOR 25058084
- ^ Lyman, Theodore (1914), "An Extension of the Spectrum in the Extreme Ultra-Violet", Nature 93: 241, Bibcode 1914Natur..93..241L, doi:10.1038/093241a0
- ^ Balmer, J. J. (1885), "Notiz uber die Spectrallinien des Wasserstoffs", Annalen der Physik 261 (5): 80–87, Bibcode 1885AnP...261...80B, doi:10.1002/andp.18852610506, http://www3.interscience.wiley.com/journal/112487600/abstract
- ^ Paschen, Friedrich (1908), "Zur Kenntnis ultraroter Linienspektra. I. (Normalwellenlängen bis 27000 Å.-E.)", Annalen der Physik 332 (13): 537–570, Bibcode 1908AnP...332..537P, doi:10.1002/andp.19083321303, http://www3.interscience.wiley.com/journal/112500956/abstract
- ^ Brackett, Frederick Sumner (1922), "Visible and infra-red radiation of hydrogen", Astrophysical Journal 56: 154, Bibcode 1922ApJ....56..154B, doi:10.1086/142697
- ^ Pfund, A. H. (1924), "The emission of nitrogen and hydrogen in infrared", J. Opt. Soc. Am. 9 (3): 193–196, doi:10.1364/JOSA.9.000193
- ^ Humphreys, C.J. (1953), "Humphreys Series", J. Research Natl. Bur. Standards 50